The Belousov-Zhabotinsky (BZ) reaction is named after B.
P. Belousov who discovered the reaction and A.
M. Zhabotinsky who continued Belousov´s early work.
The mechanism of this oscillating reaction was published in 1972
by R.
J. Field, Endre Körös, and R.
M. Noyes. The work by Field, Körös and Noyes opened
an entire new research area: nonlinear chemical dynamics.
In its classical form the BZ reaction consist of a one-electron
redox catalyst, an organic substrate that can be easily brominated
and oxidized, and bromate ion in form of NaBrO3 or
KBrO3 all dissolved in sulfuric or nitric acid. As
catalysts mostly Ce(III)/Ce(IV) salts, Mn(II) salts and ferroin
are used. Also Ruthenium complexes are now extensively studied,
because of the reaction´s sensitivity against light and
light perturbations. As organic substrate normally malonic acid
(HOOC-CH2-COOH, MA) is used, instead of Belousov´s
original citric acid. Alkyl-substituted malonic acids have been
found useful in mechanistic studies, particularily by NMR.
Study of the BZ reaction in open or closed well-stirred
reactors, where the system can show oscillations between
an oxidized and reduced state.
The two pictures on the left and right shows the malonic acid BZ reaction in a stirred beaker (closed reactor) with ferroin as a catalyst. Ferroin, in its oxidized state, has a blue color, while in its reduced state ferroin is red. As the BZ reactions alternates between the oxidized state (left picture) and reduced state (right picture), the solution changes regularily its color. The period length of BZ systems ranges - dependent on the amounts of mineral acid, organic substrate, catalyst, bromate ion, and, last but not least, temperature, - from a fraction of a minute up to many minutes.
A particular spectacular experiment in a stirred system can be done when Ruthenium-ion is used as a catalyst and the solution is illuminated with UV light in a dark room. In its reduced state, Ru(II), has a red color, which shows an intense fluorescence. The oxidized form of Ruthenium, Ru(III), is green in color, but shows no fluorescence. Normally, when no UV source is present, the oscillatory Ru-BZ system changes color between green and red (for nice pictures of Ru(III)/Ru(II) by Prof. Matsumura´s group click here). In the presence of UV-illumination, the oscillatory Ru-BZ reactions shows a pulsating fluorescence, as indicated by the two pictures shown below.
Oscillatory fluorescence in the UV-illuminated Ru-BZ reaction.
The UV source is placed to the right of the beaker/magnetic stirrer.
The red light to the left is an (uncovered) indicator light from
the magnetic stirrer.
Other catalysts often used are Ce(IV)/Ce(III) salts or Mn(II)
salts. In case of cerium, the system oscillates between yellow
Ce(IV) and colorless Ce(III). In case of manganese the BZ reaction
switches between the red Mn(III) ion and the colorless Mn(II).
Because the color changes in cerium or manganese systems are not
as spectacular as in case of ferroin, these systems are generally
studied by electrochemical or spectrophotometric methods.
Recipe for Oscillations in a well-stirred Beaker: 0.3 M malonic acid, 0.1 M NaBrO3 (or KBrO3, but note that NaBrO3 is more soluble and preferred when using larger initial BrO3- concentrations) 2 x 10-3 M (NH4)2Ce(NO3)6 (you can also use chloride-free ferroin or MnSO4) all in 1 M sulfuric acid. Dissolve the chemicals in the following order in the 1 M H2SO4: malonic acid, Ce(IV), and finally bromate. After an induction period of several minutes the oscillations start and last for approximately 1 hour.
Study of the BZ reaction in a thin unstirred layer of reacting solution, where concentric waves ("target patterns") or spiral waves are developed. This system is almost exclusively studied with ferroin and malonic acid as substrates. Note that chloride ions have to be avoided because they may act as inhibitors. The reacting solution is normally spread out as a thin film with a few millimeters thickness in a petri dish (diameter ca. 10 cm). After a certain time blue oxidation fronts which propagate on the red background (reduced ferroin) develop.
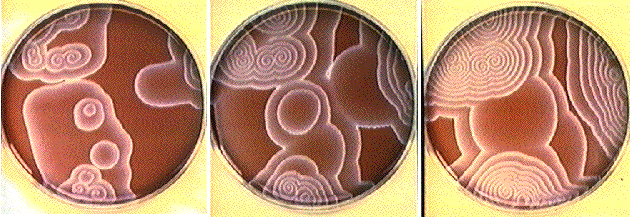
From left to right: Propagating oxidation waves in an unstirred
layer of the ferroin-malonic acid BZ reaction. When the wave is
broken at a certain point (for example by a gentle airflow through
a pipette) a pair of spiral waves develop at this point.